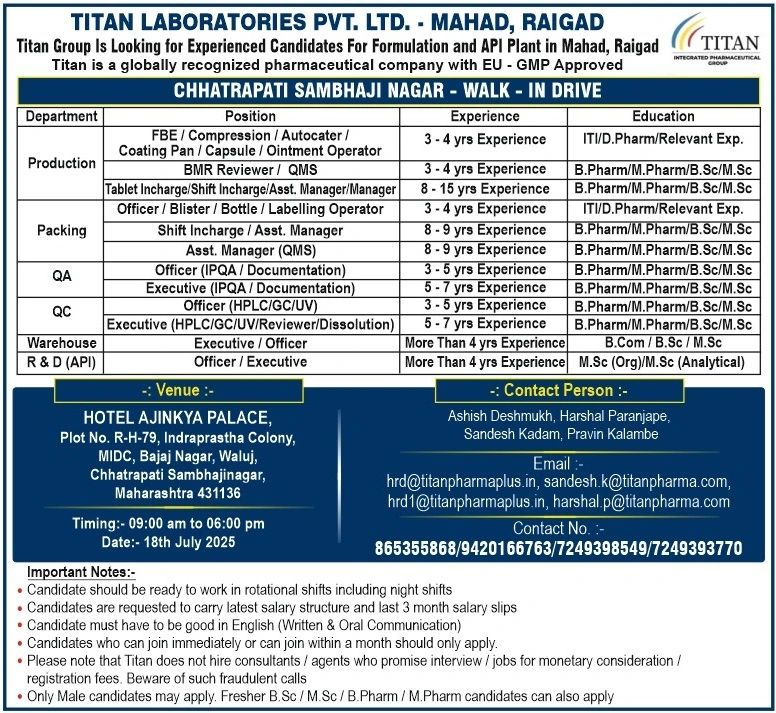
Titan Laboratories Pvt. Ltd., an EU-GMP approved pharmaceutical company specializing in modified-release pellets, is conducting a walk-in drive for experienced professionals at its Mahad, Raigad facility.
Walk-In Interview Details
📅 Date: 18th July 2025 (Friday)
⏰ Time: 9:00 AM – 6:00 PM
📍 Venue:
Hotel Ajinkya Palace, Plot No. R-H-79,
Indraprastha Colony, MIDC, Bajaj Nagar,
Waluj, Chhatrapati Sambhajinagar,
Maharashtra – 431136
📧 Email CV: hrd@titanpharmaplus.in / sandesh.k@titanpharma.com
📞 Contact: 865355868, 9420166763
Open Positions
| Department | Role | Qualification | Experience |
|---|---|---|---|
| Production | Operators/Supervisors | ITI/D.Pharm/B.Pharm | 3-15 years |
| Quality Assurance | IPQA/QMS Officers | B.Pharm/M.Pharm | 3-9 years |
| Quality Control | HPLC/GC Analysts | M.Sc/B.Pharm | 3-7 years |
| Warehouse | Officers/Executives | B.Com/B.Sc | 4+ years |
| R&D (API) | Research Officers | M.Sc (Organic) | 4+ years |
Eligibility Criteria
- Education: ITI/D.Pharm/B.Pharm/M.Pharm/M.Sc
- Experience: 3-15 years in pharma manufacturing
- Language: Proficient English (written & spoken)
- Shift: Willingness for rotational/night shifts
Mandatory Documents
- Updated resume
- Last 3 months’ salary slips
- Educational certificates
- Aadhar & PAN card copies
Why Join Titan Laboratories?
- EU-GMP Certified Facility
- specialist in Modified-Release Formulations
- Career Growth Opportunities
- Strategic Location (Mumbai-Goa Highway)
Interview Preparation Tips
- Highlight GMP/technical skills relevant to your department
- Prepare for practical knowledge tests (especially for QC roles)
- Bring complete documentation for immediate processing
About Titan Laboratories
Specialization: Sustained-release pellets, APIs
Facility Location: Mahad, Raigad (180km from Mumbai)
Approvals: EU-GMP, WHO-GMP
Source: Titan Laboratories Website